|
Foodlaw-Reading
Dr David Jukes, The University of
Reading, UK
Providng access to food law since May 1996 |

|
.....  ..... ..... ..... .....  ..... .....  ..... .....  ..... ..... 
|
Last updated:
3 October, 2023
GMOs, NGTs and PBOs
Providing access to EU and UK legislation
GMOs = Genetically modified organisms / NGTs = Novel Genomic Techniques (EU term) / PBOs - Precision Bred Organism (UK term)
Summary
Note: This page considers the EU legislation on genetically modified (GM) foods and the more recent developments involving Novel Genomic Techniques (NGTs) (the EU tern) and Precision Bred Organisms (PBOs) (the UK term). Originally the controls were based on those applied to 'novel foods'. However in 2003 separate EU legislation was adopted on GM food. For details of legislation on 'novel foods', see the other page: Novel Food Legislation in the EU. The following diagram illustrates the development of both the GM controls and those for 'novel foods':
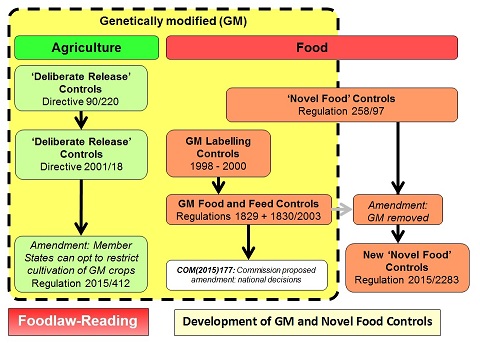
For a larger version of this figure, see: Diagram: GM and Novel Foods
Initial controls for the approval and use of genetically modified materials in agriculture were established by Directive 90/220 on the deliberate release into the environment of genetically modified organisms. These were subsequently updated and replaced by Directive 2001/18/EC on the deliberate release into the environment of genetically modified organisms and repealing Directive 90/220/EEC.
The use of GM material for food was initially covered by Regulation 258/97 of the European Parliament and of the Council concerning novel foods and novel food ingredients which came into effect on the 15 May 1997 - foods used before this date would not be considered 'novel'. This contained provisions for the approval of GM material as food and for the possible requirement for labelling. However, before May 1997 two GM products (one soya and one maize) had been approved for use under Directive 90/220 and could not therefore be subject to the rquirements of Regulation 258/97 (since, by definition, they could not be considered 'novel'). Special controls were therefore adopted by Regulation 1813/97 to impose the same requirements for labelling on these two GM products as would have been required under the novel food controls. Howver as imports were just beginning to arrive, consumer concerns increased and additional special labelling rules were introduced, first by Regulation 1139/98 and then by Regulation 49/2000.
Major changes were introduced in 2003 with the adoption of specific controls applying to both food and feed - Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed. This removed GM food from the definition of 'novel food' and contained updated procedures for the approval of GM food and for the labelling of products derived from GM material.
Approval of GM material for food use has been controversial and Member States are divided on whether they should be used for food. Although the European Food Safety Authority has generally issued positive opinions on their safety (itself also controversial), Member States have subsequently failed to agree and adopt the necessary legal documents. Regulation 1829/2003 (and related procedural regulations) allows the Commission to adopt the approval if Member States fail to reach agreement within specified time limits and this has been the route for the adoption of most approvals. Similar issues have arisen under Directive 2001/18 but an amendment (by Directive 2015/412) allows Member States to restrict use in agriculture on their territory. To try and resolve the problem for food and feed, the Commission proposed (in COM(2015)177) a similar amendment to Regulation 1829/2003 allowing Member States to prohibit (under specified conditions) the use of approved GM foods in their territory. This though was rejected by the European Parliament; as the Commission did not withdraw the proposal, it is officially still awaiting the Council to reach a position at first readiing.
A case (C-528/16) was brought before the Court of Justice to clarify whether the more recent scientific techniques used to modify genes within a plant were classified as genetic modification under the EU legislation. The ruling, in July 2018, determined that such techniques, commonly known as 'gene editing' (GE) were covered by the detailed controls in Directive 2001/18. Debate has therefore been taking place to determine whether to amend the legislation so as to remove GE plants from the definitions within the Directive. In April 2021 the Commission started an 'open debate' and in September 2021 started the process of consultation on a new approach. In July 2023 the Commission published a proposal for a regulation on plants obtained by certain new genomic techniques and their food and feed, COM(2023)411. Similar initiatives have taken place within the UK. In March 2023, the Genetic Technology (Precision Breeding) Act 2023 was passed. Discussions on the develoopment of supporting regulations are now taking place. For updates on the EU and UK developments proposal, see the sections below - EU and NGTs / UK and PBOs.
For the Commission's page on this topic, see: Genetically Modified Organisms.
EU Legislation
- Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the deliberate release into the environment of genetically modified organisms and repealing Council Directive 90/220/EEC
(OJ L106, 17.4.2001, page 1) See also: Commission Declaration (OJ L106, 17.4.2001, page 39)
Amendments
- Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed (OJ L268, 18.10.2003, page 1)
- Regulation (EC) No 1830/2003 of the European Parliament and of the Council of 22 September 2003 concerning the traceability and labelling of genetically modified organisms and the traceability of food and feed products produced from genetically modified organisms and amending Directive 2001/18/EC
(OJ L268, 18.10.2003, page 24)
- Directive 2008/27/EC of the European Parliament and of the Council of 11 March 2008 amending Directive 2001/18/EC on the deliberate release into the environment of genetically modified organisms, as regards the implementing powers conferred on the Commission
- Directive (EU) 2015/412 of the European Parliament and of the Council of 11 March 2015 amending Directive 2001/18/EC as regards the possibility for the Member States to restrict or prohibit the cultivation of genetically modified organisms (GMOs) in their territory (OJ L68, 13.3.2015, page 1)
- Commission Directive (EU) 2018/350 of 8 March 2018 amending Directive 2001/18/EC of the European Parliament and of the Council as regards the environmental risk assessment of genetically modified organisms (OJ L67, 9.3.2018, page 30)
- Regulation (EU) 2019/1243 of the European Parliament and of the Council of 20 June 2019 adapting a number of legal acts providing for the use of the regulatory procedure with scrutiny to Articles 290 and 291 of the Treaty on the Functioning of the European Union (OJ L198, 25.7.2019, page 241)
- Regulation (EU) 2019/1381 of the European Parliament and of the Council of 20 June 2019 on the transparency and sustainability of the EU risk assessment in the food chain and amending Regulations (EC) No 178/2002, (EC) No 1829/2003, (EC) No 1831/2003, (EC) No 2065/2003, (EC) No 1935/2004, (EC) No 1331/2008, (EC) No 1107/2009, (EU) 2015/2283 and Directive 2001/18/EC (OJ L231, 6.9.2019, page 1) [These amendments take effect from 27 March 2021]
- Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed
(OJ L268, 18.10.2003, page 1)
Amendments
- Commission Regulation (EC) No 1981/2006 of 22 December 2006 on detailed rules for the implementation of Article 32 of Regulation (EC) No 1829/2003 of the European Parliament and of the Council as regards the Community reference laboratory for genetically modified organisms (OJ L368, 23.12.2006, page 99)
- Regulation (EC) No 298/2008 of the European Parliament and of the Council of 11 March 2008 amending Regulation (EC) No 1829/2003 on genetically modified food and feed, as regards the implementing powers conferred on the Commission (OJ L97, 9.4.2008, page 64)
- Regulation (EU) 2019/1381 of the European Parliament and of the Council of 20 June 2019 on the transparency and sustainability of the EU risk assessment in the food chain and amending Regulations (EC) No 178/2002, (EC) No 1829/2003, (EC) No 1831/2003, (EC) No 2065/2003, (EC) No 1935/2004, (EC) No 1331/2008, (EC) No 1107/2009, (EU) 2015/2283 and Directive 2001/18/EC (OJ L231, 6.9.2019, page 1) [These amendments take effect from 27 March 2021]
- Regulation (EC) No 1830/2003 of the European Parliament and of the Council of 22 September 2003 concerning the traceability and labelling of genetically modified organisms and the traceability of food and feed products produced from genetically modified organisms and amending Directive 2001/18/EC
(OJ L268, 18.10.2003, page 24)
Amendments
- Regulation (EC) No 1137/2008 of the European Parliament and of the Council of 22 October 2008 adapting a number of instruments subject to the procedure laid down in Article 251 of the Treaty to Council Decision 1999/468/EC, with regard to the regulatory procedure with scrutiny — Adaptation to the regulatory procedure with scrutiny — Part One (OJ L311, 21.11.2008, page 1)
- Regulation (EU) 2019/1243 of the European Parliament and of the Council of 20 June 2019 adapting a number of legal acts providing for the use of the regulatory procedure with scrutiny to Articles 290 and 291 of the Treaty on the Functioning of the European Union (OJ L198, 25.7.2019, page 241) [Provides increased authority to the Commission to adopt delegated legislation]
- Regulation (EC) No 1946/2003 of the European Parliament and of the Council of 15 July 2003 on transboundary movements of genetically modified organisms ( OJ L 287, 5.11.2003, page 1)
Consolidated Texts:
Approvals
Proposed EU Controls for Novel Genomic Techniques (NGTs) (Proposal published July 2023)
For the Commission's Proposal, see:
For News Items, see:
- 24 August 2023 - Council discussions: Regulation on plants obtained by certain new genomic techniques and food and feed products
- 25 July 2023 - Remarks by Commissioner Stella Kyriakides at the Agrifish Council - New Genomic Techniques
- 7 July 2023 - Commission consultation: Legislation for plants produced by certain new genomic techniques
- 5 July 2023 - FDE Comments: EU paves way for new genomic techniques
- 5 July 2023 - Commission announcement: European Green Deal: more sustainable use of plant and soil natural resources
- 24 September 2021 - Commission consultation: Legislation for plants produced by certain new genomic techniques
- 27 May 2021 - Council discussions: New genomic techniques
- 29 April 2021 - Biotechnologies: Commission seeks open debate on New Genomic Techniques as study shows potential for sustainable agriculture and need for new policy
For the Commission's page on this issue, see: New techniques in biotechnology
UK Legislation
Brexit: Prior to the IP Completion Day (31 December 2020), the legal requirements given in the EU Regulations listed above still applied to the UK. Since IP Completion Day, the EU Regulations above have been incorporated into UK legislation but with amendments to correct deficiencies. Information on this is given below. For more details of the process of incorporating EU legislation into UK law, see the separate page: UK Food Law: EU Legislation as Amended for the UK. Provisions for the enforcement of the controls (originally the EU Regulations but now as amended) have been provided in the UK Regulations listed below. For Northern Ireland, EU rules still apply.
Guidance:
EU Legislation with links to legislation.gov.uk: amended for application in Great Britain:
- Regulation (EC) No. 1829/2003 of the European Parliament and of the Council on genetically modified food and feed as amended by:
- Genetically Modified Food and Feed (Amendment etc.) (EU Exit) Regulations 2019 (SI 2019, No. 705)
- Food and Feed Hygiene and Safety (Miscellaneous Amendments) (EU Exit) Regulations 2019 (SI 2019, No. 1013) [Revoked by 2020, No. 1504]
- Food and Feed Hygiene and Safety (Miscellaneous Amendments etc.) (EU Exit) Regulations 2020 (SI 2020, No. 1504)
- Food and Feed (Miscellaneous Amendments) Regulations 2022 (SI 2022, No. 1351)
- Regulation (EC) No 1830/2003 of the European Parliament and of the Council concerning the traceability and labelling of genetically modified organisms and the traceability of food and feed products produced from genetically modified organism as amended by:
- Genetically Modified Organisms (Amendment) (EU Exit) Regulations 2019 (SI 2019, No. 90)
as amended by:
- Environment, Food and Rural Affairs (Amendment) (EU Exit) Regulations 2019 (SI 2019, No. 778)
- Genetically Modified Organisms (Amendment) (EU Exit) Regulations 2020 (SI 2020, No. 1421)
- Regulation (EC) No 1946/2003 of the European Parliament and of the Council on transboundary movements of genetically modified organism - see 2019, No 90
- Genetically Modified Organisms (Amendment) (EU Exit) Regulations 2019 (SI 2019, No. 90)
as amended by:
- Genetically Modified Organisms (Amendment) (EU Exit) Regulations 2020 (SI 2020, No. 1421)
UK Legislation: with links to legislation.gov.uk
Note: Since the European controls on deliberate release were adopted as a Directive, all legal requirements were already contained in UK legislation. The following UK Regulations contain both the technical requirements and the enforcement provisions for deliberate release..
- England
- Genetically Modified Organisms (Deliberate Release) Regulations 2002
(SI 2002, No. 2443)
[Revised] as amended by:
- Genetically Modified Organisms (Deliberate Release) (Amendment) Regulations 2004 (SI 2004, No. 2411)
- Medicines (Marketing Authorisations Etc.) Amendment Regulations 2005 (SI 2005, No. 2759)
- Human Fertilisation and Embryology (Consequential Amendments and Transitional and Saving Provisions) Order 2009 (SI 2009, No. 1892)
- Environment, Food and Rural Affairs (Miscellaneous Amendments) (England) Regulations 2018 (SI 2018, No. 575)
- Genetically Modified Organisms (Amendment) (England) (EU Exit) Regulations 2019 (SI 2019, No. 88)
as amended by:
- Genetically Modified Organisms (Deliberate Release) (Amendment) (England) Regulations 2019 (SI 2019, No. 1252)
- Food and Farming (Amendment) (EU Exit) Regulations 2019 (SI 2019, No. 759)
- Food and Farming (Amendment) (EU Exit) Regulations 2019 (SI 2019, No. 759)
- Genetically Modified Organisms (Deliberate Release) (Amendment) (England) Regulations 2019 (SI 2019, No. 1252)
- Wales
- Genetically Modified Organisms (Deliberate Release) (Wales) Regulations 2002
(SI 2002, No. 3188 (W.304))
[Revised]
as amended by:
- Genetically Modified Organisms (Deliberate Release) (Wales) (Amendment) Regulations 2005 (SI 2005, No. 1913 (W.156))
- Medicines (Marketing Authorisations Etc.) Amendment Regulations 2005 (SI 2005, No. 2759)
- Natural Resources Body for Wales (Functions) Order 2013 (2013 No. 755 (W. 90)) [Note: very long document]
- Environment, Planning and Rural Affairs (Miscellaneous Amendments) (Wales) Regulations 2018 (2018 No. 1216 (W. 249))
- Genetically Modified Organisms (Deliberate Release and Transboundary Movement) (Miscellaneous Amendments) (Wales) (EU Exit) Regulations 2019 (2019 No. 379 (W. 94)) as amended by:
- Genetically Modified Organisms (Deliberate Release and Transboundary Movement) (Miscellaneous Amendments) (Wales) (EU Exit) (No. 2) Regulations 2019 (2019 No. 1492 (W. 271))
- Rural Affairs, Environment, Fisheries and Food (Miscellaneous Amendments and Revocations) (Wales) Regulations 2019 (2019 No. 463 (W. 111))
- Genetically Modified Organisms (Deliberate Release) (Amendment) (Wales) Regulations 2019 (SI 2019, No. 1316 (W.228))
- Genetically Modified Organisms (Deliberate Release) (Amendment) (Wales) (Amendment) Regulations 2019 (SI 2019, No. 1407 (W.248)) [Correcting errors]
- Genetically Modified Organisms (Deliberate Release and Transboundary Movement) (Miscellaneous Amendments) (Wales) (EU Exit) (No. 2) Regulations 2019 (2019 No. 1492 (W. 271))
- Scotland
- Genetically Modified Organisms (Deliberate Release) (Scotland) Regulations 2002 (SSI 2002, No. 541)
as amended by:
- Genetically Modified Organisms (Deliberate Release) (Scotland) Amendment Regulations 2004 (SSI 2004, No. 439)
- Medicines (Marketing Authorisations Etc.) Amendment Regulations 2005 (SI 2005, No. 2759)
- Food (Scotland) Act 2015 (Consequential and Transitional Provisions) Order 2015 (SSI 2015, No. 100)
- Genetically Modified Organisms (EU Exit) (Scotland) (Amendment) Regulations 2019 (SSI 2019 No. 57)
- Genetically Modified Organisms (Deliberate Release etc.) (Miscellaneous Amendments) (Scotland) Regulations 2019 (SSI 2019, No. 86)
- Northern Ireland
- Genetically Modified Organisms (Deliberate Release) Regulations (Northern Ireland) 2003
(SRNI 2003, No. 167)
as amended by:
- Genetically Modified Organisms (Deliberate Release) (Amendment) Regulations (Northern Ireland) 2003 (SRNI 2003, No. 206)
- Genetically Modified Organisms (Deliberate Release) (Amendment) Regulations (Northern Ireland) 2005 (SRNI 2005, No. 272)
- Pesticides, Genetically Modified Organisms and Fertilisers (Miscellaneous Amendments) Regulations (Northern Ireland) 2018 (SRNI 2018, No. 188)
- Genetically Modified Organisms (Amendment) (Northern Ireland) (EU Exit) Regulations 2019 (SI 2019, No. 190)
as amended by:
- Genetically Modified Organisms (Amendment)(EU Exit) Regulations (Northern Ireland) 2020 (SRNI 2020 No. 269)
- Food and Farming (Amendment) (EU Exit) Regulations 2019 (SI 2019, No. 759)
- Genetically Modified Organisms (Deliberate Release) (Amendment) Regulations (Northern Ireland) 2019 (SRNI 2019, No. 223)
- Food and Farming (Amendment) (EU Exit) Regulations 2019 (SI 2019, No. 759)
- Genetically Modified Organisms (Amendment)(EU Exit) Regulations (Northern Ireland) 2020 (SRNI 2020 No. 269)
Enforcement
Requirements for implementation and enforcement of controls derived from EU Regulations are provided separately for the four parts of the United Kingdom.
- England
- Genetically Modified Food (England) Regulations 2004 (SI 2004, No. 2335)
as amended by:
- Genetically Modified Food and Feed (Amendment etc.) (EU Exit) Regulations 2019 (SI 2019, No. 705)
- Genetically Modified Organisms (Transboundary Movements) (England) Regulations 2004
(SI 2004, No. 2692)
[Revised] as amended by:
- Genetically Modified Organisms (England) (Amendments) Regulations 2008 (SI 2008, No. 2598)
- Genetically Modified Organisms (Amendment) (England) (EU Exit) Regulations 2019 (SI 2019, No. 88) as amended by:
- Genetically Modified Organisms (Deliberate Release) (Amendment) (England) Regulations 2019 (SI 2019, No. 1252)
- Genetically Modified Organisms (Traceability and Labelling) (England) Regulations 2004
(SI 2004, No. 2412) [Revised] as amended by:
- Genetically Modified Organisms (England) (Amendments) Regulations 2008 (SI 2008, No. 2598)
- Wales
- Genetically Modified Food (Wales) Regulations 2004 (SI 2004, No. 3220 (W.276))
as amended by:
- Food and Feed (Miscellaneous Amendments and Revocations) (Wales) Regulations 2018 (SI 2018 No. 806 (W. 162))
- Food and Feed Regulated Products (Miscellaneous Amendments) (Wales) (EU Exit) Regulations 2019 (SI 2019 No. 425 (W. 99))
- Food and Feed Hygiene and Safety (Miscellaneous Amendments and Saving Provision) (Wales) (EU Exit) Regulations 2020 (SI 2020 No. 1581 (W. 331))
- Genetically Modified Organisms (Traceability and Labelling) (Wales) Regulations 2005 (SI 2005 No. 1914 (W.157))
[Revised] as amended by:
- Rural Affairs, Environment, Fisheries and Food (Miscellaneous Amendments and Revocations) (Wales) Regulations 2019 (2019 No. 463 (W. 111))
- Genetically Modified Organisms (Transboundary Movement) (Wales) Regulations 2005 (SI 2005, No. 1912 (W.155))
[Revised] as amended by:
- Genetically Modified Organisms (Deliberate Release and Transboundary Movement) (Miscellaneous Amendments) (Wales) (EU Exit) Regulations 2019 (2019 No. 379 (W. 94)) as amended by:
- Genetically Modified Organisms (Deliberate Release and Transboundary Movement) (Miscellaneous Amendments) (Wales) (EU Exit) (No. 2) Regulations 2019 (2019 No. 1492 (W. 271))
- Scotland
- Genetically Modified Food (Scotland) Regulations 2004 (SSI 2004, No. 432)
as amended by:
- Food (Scotland) Act 2015 (Consequential and Transitional Provisions) Order 2015 (SSI 2015, No. 100)
- Food and Feed Safety and Hygiene (EU Exit) (Scotland) (Amendment) Regulations 2019 (SSI 2019 No. 52) as amended by:
- Food and Feed (EU Exit) (Scotland) (Amendment) Regulations 2020 (SSI 2020 No. 372) as amended by:
- Animals, Food and Feed (EU Exit) (Scotland) (Amendment) Regulations 2020 (SSI 2020 No. 455)
- Genetically Modified Organisms (Traceability and Labelling) (Scotland) Regulations 2004
(SSI 2004, No. 438) [Revised]
as amended by:
- Genetically Modified Organisms (Deliberate Release etc.) (Miscellaneous Amendments) (Scotland) Regulations 2019 (SSI 2019, No. 86)
- Feed (Transfer of Functions) (Miscellaneous Amendments) (Scotland) Regulations 2020 (SSI 2020 No. 467)
- Genetically Modified Organisms (Transboundary Movements) (Scotland) Regulations 2005
(SSI 2005, No. 316) [Revised]
as amended by:
- Genetically Modified Organisms (EU Exit) (Scotland) (Amendment) Regulations 2019 (SSI 2019 No. 57)
- Genetically Modified Organisms (Deliberate Release etc.) (Miscellaneous Amendments) (Scotland) Regulations 2019 (SSI 2019, No. 86)
- Northern Ireland
- Genetically Modified Food Regulations (Northern Ireland) 2004 (SRNI 2004, No. 385)
as amended by:
- Food (Miscellaneous Amendments and Revocations) Regulations (Northern Ireland) 2019 (SRNI 2019 No. 5)
- Regulated Products (Amendment) (Northern Ireland) (EU Exit) Regulations 2019 (SI 2019 No. 849)
- Genetically Modified Organisms (Traceability and Labelling) Regulations (Northern Ireland) 2005
(SRNI 2005, No. 271)
as amended by:
- Pesticides, Genetically Modified Organisms and Fertilisers (Miscellaneous Amendments) Regulations (Northern Ireland) 2018 (SRNI 2018, No. 188)
- Genetically Modified Organisms (Transboundary Movements) Regulations (Northern Ireland) 2005 (SRNI 2005 No. 209) as amended by:
- Genetically Modified Organisms (Amendment) (Northern Ireland) (EU Exit) Regulations 2019 (SI 2019 No. 190) as amended by:
Precision Bred Organisms (PBOs) - UK Developments
Key documents:
For News Items (with links to some relevant documents), see:
- 21 September 2023 Discussion on Precision Breeding at the FSA Board meeting – 20 September 2023
- 7 September 2023 FSA Board Paper: Genetic Technology (Precision Breeding)
- 23 March 2023 - Genetic Technology Act key tool for UK food security [DEFRA Press Release: Act passed]
- 9 March 2023 - FSA Board Paper: The Genetic Technology (Precision Breeding) Bill: Consumer Information, Traceability and Developing Other Elements of the New Regulatory Framework for Precision-Bred Food and Animal Feed
- 31 October 2022 - Genetic Technology Bill to take on most pressing environmental problems of our time. Covers precision-bred plants and animals developed through techniques such as gene editing [DEFRA Press Release: Third Reading in House of Commons]
- 28 September 2022 - Summary of discussions at FSA Board meeting – 26 September 2022
- 30 August 2022 - FSA Board Paper: The Genetic Technology (Precision Breeding) Bill
- 25 May 2022 - Genetic Technology Bill: enabling innovation to boost food security [DEFRA Press Release: Bill introduced in House of Commons]
- 29 September 2021 - The Food Standards Agency responds to government gene editing plans
- 29 September 2021 - Plans to unlock power of gene editing unveiled: Use of gene editing technologies to be enabled to help better protect the environment [DEFRA Press Release: Consultation outcome]
- 21 July 2021 - FSA Report: UK consumers give their views on genome edited food
- 7 January 2021 - DEFRA Consultation: Gene editing creates potential to protect the nation’s environment, pollinators and wildlife
This page was first provided on 14 January 2016
To go to main Foodlaw-Reading
Index page, click here.
![]()
