Foodlaw-Reading
Dr David Jukes, The University of Reading, UK
Providng access to food law since May 1996
![]()
Foodlaw-ReadingDr David Jukes, The University of Reading, UKProvidng access to food law since May 1996 |
|
|
Last updated: 21 March, 2023
On this page:
|
After many years of discussion and dispute, EU controls were adopted in 2006 by Regulation (EC) No 1924/2006. This started to apply from July 2007 but elements became effective at various later dates and some aspects are still not operating.
The regulation provides the following definitions:
The Regulation contains an Annex which lists the permitted nutrition claims and the conditions which must be met if they are to be used. Subject to the provisions of the Regulation, these are generally available for use by any food meeting the stated conditions. Only authorised health claims can be used. The types of claims which can be authorised are set out in Articles 13 and 14 of the Regulation and it provides the procedures for gaining approval and for the establishment of a Register of Health Claims.
Since all authorised claims had to be substantiated by an assessment of the scientific evidence supporting the claim (performed by the European Food Safety Authority (EFSA)), the process of adopting the initial list of authorised claims took a considerable time. A large number of claims relating to 'botanicals' have still not been assessed. Another difficulty has been the provision, included in Article 4(1) of the Regulation, for the adoption of 'nutrient profiles' which would prevent the use of claims on foods considered to have an unhealthy profile - no profiles have yet been adopted. An overview of the process is shown in the following figure:
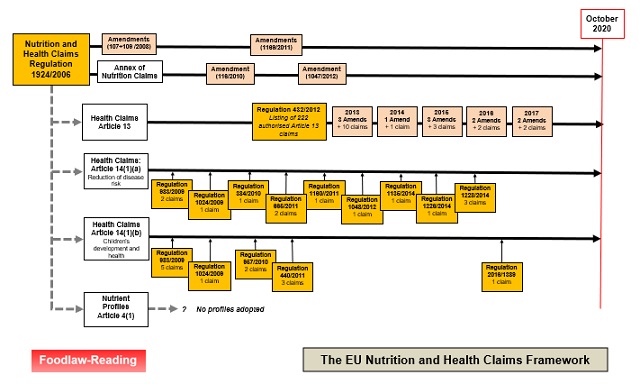
For a larger version of this figure, see: Figure: Claims
Following the departure of the UK from the European Union, the controls applied in the UK (excluding Northern Ireland)from 1 January 2021 are still based on those of the EU but adapted as separate UK legal requirements. In particular, there is now a Great Britain Register of Nutrition and Health Claims. Approval of new claims will be subject to an assessment performed by a UK based scientific committee. For more details, see UK Legislation below.
Consolidated Text: A copy of the consolidated text incorporating the amendments is available. See: Consolidated Text (December 2014)
Derogations:
For more details about the legislation, supporting documents, approvals and procedures see: EU Commission - DG Health and Food Safety - Nutrition and Health Claims
The Commission has established an on-line EU Register of Nutrition and Health Claims which shows:
|
[Note: the following lists do not include Regulations refusing to authorise claims]
Article 13 Claims - Function claims
This article provides for the approval of health claims which describe or refer to:
(a) the role of a nutrient or other substance in growth, development and the functions of the body; or
(b) psychological and behavioural functions; or
(c) without prejudice to Directive 96/8/EC, slimming or weight-control or a reduction in the sense of hunger or an increase in the sense of satiety or to the reduction of the available energy from the diet.These may be approved following a simplified procedure if they are
(i) based on generally accepted scientific evidence; and
(ii) well understood by the average consumer.The first list of authorised claims (containing 222 claims) was published in 2012 and has been amended to add additional authorisations.
A consolidated version of Regulation 432/2012 is available. See: Commission Regulation (EU) No 432/2012 (August 2017).
The Register shows a total of 242 authorised Article 13 claims (of which 1 is curretently restricted due to proprietary data) and 2,014 non-authorised Article 13 claims (March 2023)
Note: Function claims for 'botanicals' have not yet progressed and remain outside the scope of the approved register of claims. Approximately 1,500 such claims were submitted for approval.
Article 14(1)(a) - Reduction of disease risk claims
These are defined as 'any health claim that states, suggests or implies that the consumption of a food category, a food or one of its constituents significantly reduces a risk factor in the development of a human disease'. These may be approved following the application of the standard approval process. These are being approved and listed in individual Regulations as follows. Additional Regulations provide details of rejected claims - they are not listed here.
The Register shows a total of 14 authorised Article 14(1)(a) claims and 30 non-authorised Article 14(1)(a) claims (March 2023).
- Commission Regulation (EC) No 983/2009 of 21 October 2009 on the authorisation and refusal of authorisation of certain health claims made on food and referring to the reduction of disease risk and to children’s development and health (OJ L277, 22.10.2009, page 3) [Authorised 2 claims]
- Amended by Commission Regulation (EC) 376/2010 of 3 May 2010 (OJ L111, 4.5.2010, page 3)
- Amended by Commission Regulation (EU) No 686/2014 of 20 June 2014 (OJ L182, 21.6.2014, page 27)
- Commission Regulation (EC) No 1024/2009 of 29 October 2009 on the authorisation and refusal of authorisation of certain health claims made on food and referring to the reduction of disease risk and to children’s development and health (OJ L283, 30.10.2009, page 22) [Authorised 1 claim]
- Commission Regulation (EU) No 384/2010 of 5 May 2010 on the authorisation and refusal of authorisation of certain health claims made on foods and referring to the reduction of disease risk and to children’s development and health (OJ L113, 6.5.2010, page 6) [Authorised 1 claim]
- Amended by Commission Regulation (EU) No 686/2014 of 20 June 2014 (OJ L182, 21.6.2014, page 27)
- Commission Regulation (EU) No 665/2011 of 11 July 2011 on the authorisation and refusal of authorisation of certain health claims made on foods and referring to the reduction of disease risk (OJ L182, 12.7.2011, page 5) [Authorised 2 claims]
- Commission Regulation (EU) No 1160/2011 of 14 November 2011 on the authorisation and refusal of authorisation of certain health claims made on foods and referring to the reduction of disease risk (OJ L296, 15.11.2011, page 26) [Authorised 1 claim]
- Commission Regulation (EU) No 1048/2012 of 8 November 2012 on the authorisation of a health claim made on foods and referring to the reduction of disease risk (OJ L310, 9.11.2012, page 38) [Authorised 1 claim (but based on 2 opinions)]
- Commission Regulation (EU) No 1135/2014 of 24 October 2014 on the authorisation of a health claim made on foods and referring to the reduction of disease risk (OJ L307, 28.10.2014, page 23) [Authorised 1 claim]
- Commission Regulation (EU) No 1226/2014 of 17 November 2014 on the authorisation of a health claim made on foods and referring to the reduction of disease risk (OJ L331, 18.11.2014, page 3) [Authorised 1 claim]
- Commission Regulation (EU) No 1228/2014 of 17 November 2014 authorising and refusing to authorise certain health claims made on foods and referring to the reduction of disease risk (OJ L331, 18.11.2014, page 8) [Authorised 3 claims]
- Commission Regulation (EU) 2023/648 of 20 March 2023 authorising a health claim made on foods and referring to the reduction of disease risk (OJ L 81, 21.3.2023, page 8) [Authorised 1 claim based on proprietary data]
Article 14(1)(b) - Claims referring to children's development and health.
These may be approved following the application of the standard approval process. These are being approved and listed in individual Regulations as follows. Additional Regulations provide details of rejected claims - they are not listed here.
- Commission Regulation (EC) No 983/2009 of 21 October 2009 on the authorisation and refusal of authorisation of certain health claims made on food and referring to the reduction of disease risk and to children’s development and health (OJ L277, 22.10.2009, page 3) [Authorised 5 claims]
- Commission Regulation (EC) No 1024/2009 of 29 October 2009 on the authorisation and refusal of authorisation of certain health claims made on food and referring to the reduction of disease risk and to children’s development and health (OJ L283, 30.10.2009, page 22) [Authorised 1 claim]
- Commission Regulation (EU) No 957/2010 of 22 October 2010 on the authorisation and refusal of authorisation of certain health claims made on foods and referring to the reduction of disease risk and to children’s development and health (OJ L279, 23.10.2010, page 13) [Authorised 2 claims]
- Commission Regulation (EU) No 440/2011 of 6 May 2011 on the authorisation and refusal of authorisation of certain health claims made on foods and referring to children’s development and health (OJ L119, 7.5.2011, page 4) [Authorised 3 claims]
- Commission Regulation (EU) 2016/1389 of 17 August 2016 authorising a health claim made on foods and referring to children's development and health (OJ L223, 18.8.2016, page 55) [Authorised 1 claim]
The Register shows a total of 12 authorised Article 14(1)(b) claims and 46 non-authorised Article 14(1)(b) claims (March 2023 ).
Brexit: Prior to the IP Completion Day (31 December 2020), the legal requirements given in the EU Regulations listed above still applied to the UK. Since IP Completion Day, the EU Regulations above have been incorporated into UK legislation but with amendments to correct deficiencies. Information on this is given below. For more details of the process of incorporating EU legislation into UK law, see the separate page: UK Food Law: EU Legislation as Amended for the UK. Provisions for the enforcement of the controls (originally the EU Regulations but now as amended) have been provided in the UK Regulations listed below. For Northern Ireland, EU rules still apply.
Guidance and official support (from Department of Health and Social Care):
EU Legislation with links to legislation.gov.uk: amended for application in the UK:
Note: The numerous EU Regulations authorising, as listed above, or refusing to authorise health claims have also been adopted into UK legislation (and amended) but are not listed here. Search the legislation.gov.uk site for individual regulations and check SI 2019, No. 651 and SI 2020, No. 1476 for amendments.
Enforcement
Requirements for implementation and enforcement are provided separately for the four parts of the United Kingdom.
 |
This guidance was issued by the Department of Health in 2011 - it is recommended that it is used with caution and in conjuction with the main web page shown above under 'Guidance and Official Support': |
 |
General Principles on Flexibility of Wording for Health Claims (A document agreed by Member States in 2012 and provided by the Department of Health) |
 |
Nutrition Legislation Information Sheet. This document, published by the Department of Health and Social Care, is intended to help food businesses comply with nutrition legislation - some sections relate to claims. (Originally published in 2014, this is a pdf version of the updated DHSC web page from November 2022). [Provided under the Open Government Licence. The original publication accessed from: |
 |
Guidance on comparative nutrition claims (published by the UK's Food and Drink Federation in January 2018) |